TUCSON (KGUN9-TV) - The U.S. Food and Drug Administration has approved labeling changes for Essure.
Essure is a form of permanent birth control offered to women as an alternative to having your tubes tied. A doctor inserts two metal coils into a woman's Fallopian tubes causing the body to build scar tissue around the coils.
This comes after the FDA received complaints from thousands of women. In March, the FDA had more than 5,000 adverse reports from women who say the device led to health issues.
As KGUN9 reported, one Tucson woman said the device broke apart inside her. Earlier this year the FDA says it received 294 reports of pregnancy loss in women with the device which includes 96 ectopic pregnancies, 43 elective pregnancy terminations, and 155 other pregnancy losses.
The FDA began drafting the changes earlier this year. The changes include a boxed warning and patient decision checklist.
Below is an example of the boxed warning form FDA:
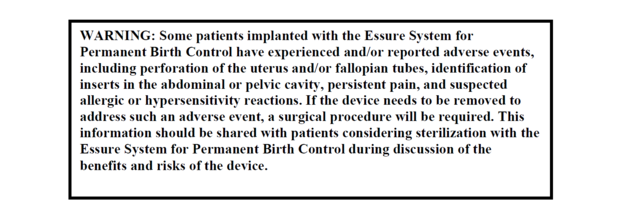
Bogle was not satisfied with the FDA approval and says she thinks Essure should be taken off of the market.
"There's no prediction of when this device is going to fail, when it's going to migrate, when it's going to perforate through something," Bogle said.
Bogle is outspoken about her experience and says her mission now is to help other women. Her advice? Do you homework when considering Essure.
"Don't do this because your doctor is pressuring you, don't do this because your family is pressuring you," Bogle said. "Just make sure it's a sure decision you want to get it and you know everything about the device."
There is a Facebook group with women who have had Essure issues that Bogle has been a part of. Bogle says there is also legislation in the works spearheaded by a Pennsylvania lawyer. The two bills, Bogle says, would make it easier for consumers to take legal action regarding medical devices and would prevent underreporting by protecting doctors if they report devices are defective.
According to it's website, Essure has been chosen by 750,000 women. The FDA says it is still a good choice for women because it is the only permanent birth control that does not require an incision or anesthesia.
Essure is produced by Bayer.
Deborah Kotz, a spokesperson for the FDA, says Bayer will send letters to health care providers about the updated labels. She says the FDA expects that the updated patient and physician labeling will be available within 30 days of the PMA supplement approval.




